The types of waste materials generated are dependant upon the type of mining activity. Around the Fal Estuary there have been effectively three main types of mining activity: (1) the recovery of cassiterite from the river sediments (so called tin streaming or placer mining); (2) the underground and opencast mining for metal (hard rock mining); and (3) opencast mining for aggregates and industrial minerals, most important of which is the mining for china clay (kaolin).
Tin streaming
During tin streaming the natural sediments containing cassiterite were extracted and then crushed and processed to recover the tin. Gerrard (2000) provides a thorough review of the tin streaming industry in southwest England. The main environmental impact of tin streaming would have been the release of large volumes of fine grained sediment which would have been carried down the adjacent river systems as suspended and also potentially as bedload material. This increase in sediment discharge down the rivers would have been made up of naturally occurring sediment which would not have had a very high metal content. Much of the tin was smelted in small local operations termed ‘blowing houses’ and these may also have released some smelt waste into the adjacent rivers and thus into the estuary.

|

|
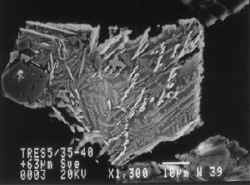
These scanning electron microscope images show grains of smelt waste recovered from the sediments in the Fal Estuary. Field of view is about 0.1 mm.
Hard rock mining
There are several different types of waste materials produced during both the hard rock mining operation itself and the subsequent mineral processing activities which aim to concentrate the minerals of value from the surrounding waste rock or gangue minerals. Close to an old mine site there may be spoil heaps composed of large blocks of rock; these spoil tips are commonly poorly vegetated as they lack soil, drain easily and may also have high levels of metal contamination either locked within mineral grains or through contamination of the water. These spoil tips are a very visual legacy of a mining operation.

Spoil tips are a very visual impact of mining as poor soil development and high levels of contamination limit natural plant growth.
A widely recognised impact of mining is on water quality where sulphide minerals are able to oxidise with the release of sulphate, commonly leading to the formation of ‘acid mine drainage’ (AMD). Although usually referred to as AMD, not all mine drainage is in fact acidic and may be either neutral or even alkaline. Associated with AMD are very high levels of metals in solution, which as conditions change downstream (principally pH) away from the mine site, may be precipitated most commonly as iron or manganese ochres Other metals such as Pb, Zn, Cu and As may also be precipitated along with the ochres. Many Cornish mines were kept dry not only by pumping but also via complex networks of drainage tunnels or adits which discharged mine waters directly into river courses and or into the sea. The blockage and subsequent failure of one of these adits led to the most famous mine water pollution incident to have affected the Fal Estuary in recent years (a detailed account of this can be found in the Wheal Jane Incident section). Metals initially released in solution can become absorbed within and adsorbed onto the surfaces of other minerals such as clays; the metals are relatively loosely attached to these minerals and if the surrounding water chemistry changes they may be re-released in solution.
In addition to the release of metals in solution, a long-lived source of mine waste pollution is the release of fine grained particulate material, which typically comprises the waste mineral phases, or grains of the processed ore minerals which were too fine grained to be successfully recovered during the processing of the ore. In modern mining operations fine grained mine waste, or tailings, is allowed to settle out within large-scale tailings dams as seen at the Wheal Jane tailings dam at Bissoe.

The Wheal Jane tailings pond at Bissoe can be seen in the top left of this image, whilst in the valley floor is the Wheal Jane mine water treatment plant.
However, in the historical past the mine waste tailings were commonly discharged into the adjacent river systems where the mineral particles were transported as bedload and suspended load. On entering the larger estuarine water bodies the mixing of fresh and saline waters, along with the deceleration of the flow velocity of the stream entering the larger body of water caused the deposition of this particulate waste. Consequently the release of this particulate mine waste led to extensive siltation; where undisturbed by later human activity the sediment retains a geochemical and mineralogical signature of the historical impact of mining on the environment. The scale of the release of particulate mine waste can be illustrated by the early observations reported by Whitley (1881):
‘at higher Carnon [on the Carnon River which drains into Restronguet Creek] a foot bridge which in 1815 to 1820 was three to four feet above the level of the stream, in 1867 had been buried under about two feet of detritus’
This quotation suggests that there was an average siltation rate of 3-4 mm per year in this area. The particulate mine waste commonly contains the economically important ore minerals which were being mined. These minerals may occur as discrete, liberated grains, which are typically very small, or as larger crystals locked within a larger fragment made up of the gangue minerals or host rock.

|

|
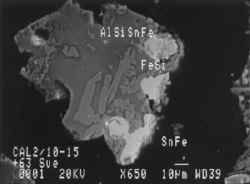
Particulate (tailings) waste makes up a significant component of the estuary sediments in the Fal. This typically includes grains of the economically valuable minerals which were not successfully recovered during mineral processing.
The average size of liberated ore minerals present reflects the efficiency of the mineral processing operation at that time. For example, Thomas (1913) estimated that about 30% of the tin mined at that time was lost. Grains of tin, less than 0.01 mm across could not be recovered during mineral processing. Although the released fine-grained tailings were the waste produced during mining and mineral processing they are still very enriched in metals in comparison with natural sediments, so much so that it has been common practice for old tailings dumps to be successfully re-mined when either the value of the mineral has increased or the technical ability to concentrate the mineral has improved. Usually the release of a relatively small volume of tailings will have a very clear impact upon the chemistry of the sediments.
The long-term fate of this particulate waste depends upon the chemistry of the environment around the particle and also the mineralogy of the particle. For example, the main mined ore mineral for tin is the oxide cassiterite (SnO2). This mineral is chemically stable; the tin is effectively locked within the mineral and is unlikely to change with time; importantly the tin is not therefore available for uptake by plants or animals. In contrast, other minerals such as pyrite (FeS2) and chalcopyrite (CuFeS2 - the main ore mineral for copper in Cornwall) are sulphides, which can oxidise. In a wet oxygenated environment pyrite will oxidise to produce sulphuric acid and iron hydroxide as follows (Merefield 1995):
2FeS2 + 7O2 + 2H2O ® 2FeSO4 + 2H2SO4
Bacteria (e.g. Thiobacillus ferroxidans) can speed up this chemical process by as much as 100 times:
4FeSO4 + O2 + 2H2SO4 ® 2Fe(SO4)3 + 2H2O
At pH values of between 4 and 7 the ferric ions produced in this reaction will tend to precipitate out as ochres. Additionally, the sulphuric acid and ferric sulphate produced from pyrite can then oxidise other metal sulphides such as chalcopyrite:
CuFeS2 + Fe(SO4)3 ® CuSO4 + 2FeSO4 + 2S
If the sulphide minerals are rapidly deposited within the estuarine sediments, which at depth lack free oxygen, then the minerals are chemically stable. If these grains are reworked and exposed to oxygen then they will start to chemically alter.
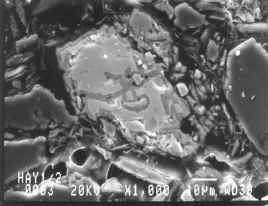
This scanning electron microscope image shows the problems which can occur when sulphide minerals are allowed to oxidise. The bright grain is the copper mineral chalcopyrite which is breaking down around the grain edges and releasing Cu in solution. Field of view is about 0.1 mm.
In addition to this type of particulate waste, man-made smelt waste products were also locally dumped both within river systems and into the estuary. Indeed in some areas of Cornwall, this material has been used in the construction of bridges, quays and even houses.
In summary, hard rock mining can result in the release of:
Metals in solution - so called ‘acid mine drainage’. Some of this metal initially released in solution can be attached through adsorption or absorption onto other mineral surfaces (e.g. on clay minerals)
Acid mine drainage can result locally in the precipitation of Fe or Mn ochres which may also contain significant concentrations of other metals
The release of fine grained particulate mine waste including grains of ore minerals which are either locked within larger fragments due to inefficient grinding of the sample during processing or alternatively very fine grains of the ore minerals which are too small to be recovered during mineral processing
The local dumping of coarse mine waste rock piles
Man-made smelt and slag products formed during the metallurgical processing of the ore.
In the Fal Estuary, the release of acid mine drainage is still an ongoing environmental issue (see section on Wheal Jane), but by far the largest impact was through the release of particulate mine waste (tailings) which were deposited throughout the estuary and today form a significant part of the intertidal mudflats and indeed, the subtidal seafloor sediments.
Opencast mining (quarrying) for aggregates and industrial minerals
When considering the environmental impact of mining on the Fal Estuary, the most important quarrying activity was the production of china clay (kaolinite), principally from the St, Austell area. Historically, china clay mining resulted in the release of both coarse sand as bedload sediment within rivers and also some fine china clay being released in suspension. The early working of china clay was by hand, the river sediment was removed, and then diverted river water was used to separate the coarse sand from the clay, fine sand and mica; a process referred to as ‘breaking in the stream’. The coarse sand settled and was subsequently removed and dumped elsewhere, whilst the remaining clay, fine sand and mica was allowed to settle. The waste sand and mica, along with unrecovered clay was then discharged into the adjacent river catchment. The early study by Everard (1962) on St. Austell Bay clearly demonstrated the impact of the release of china clay into fluvial systems and then out to sea. Indeed the Kernick River was usually referred to as the White River due to its load of china clay.

Aerial photograph of the intertidal sediments at Ardevera on the River Fal. The distinctive white colour is due to the accumulation of china clay waste.
The problem of the release of china clay waste was recognised as early as 1910 when the River Fal became highly polluted and a tax was suggested (Barton, 1966). The discharge of this mine waste into local watercourses continued until 1968 when new legislation caused the practice to stop. Although the discharge of china clay waste no longer occurs, some of the intertidal areas of the estuary are composed of this china clay waste. The release of particulate waste from this mining activity would not cause a significant increase in the amount of heavy metals in the environment. However, this sediment may contain so called ‘heavy minerals’ which are present within the granite, but subsequently concentrated during the mining activity and the release of this waste material into the rivers. Minerals which may be concentrated in this way would include zircon, monazite and xenotime which geochemically would give an increase in the abundance of Zr, Ce and La and Y respectively. Typically the release of waste from the quarrying of china clay can best be recognised by identifying the minerals present in the clay grain size fraction in the sediment in the estuaries. The natural ‘background’ clay mineralogy should be composed of the clay minerals illite and chlorite derived from the breakdown of Devonian metasedimentary rocks along with some kaolinite derived from the granites; any significant increases in the amount of kaolinite relative to illite and chlorite probably reflects the input of waste from the extraction of china clay.